Unit 2 - Notes
Unit 2: Lasers and Applications
1. Fundamentals of Laser
LASER stands for Light Amplification by Stimulated Emission of Radiation. It is a device that generates an intense beam of coherent, monochromatic, and highly directional light.
Energy Levels in Atoms
According to quantum mechanics, electrons in an atom exist in discrete energy states or levels:
- Ground State (): The lowest energy state where the atom is most stable.
- Excited State (): Higher energy states. Atoms in these states are unstable and tend to return to the ground state.
Interaction of Radiation with Matter
There are three distinct processes by which radiation (photons) interacts with matter:
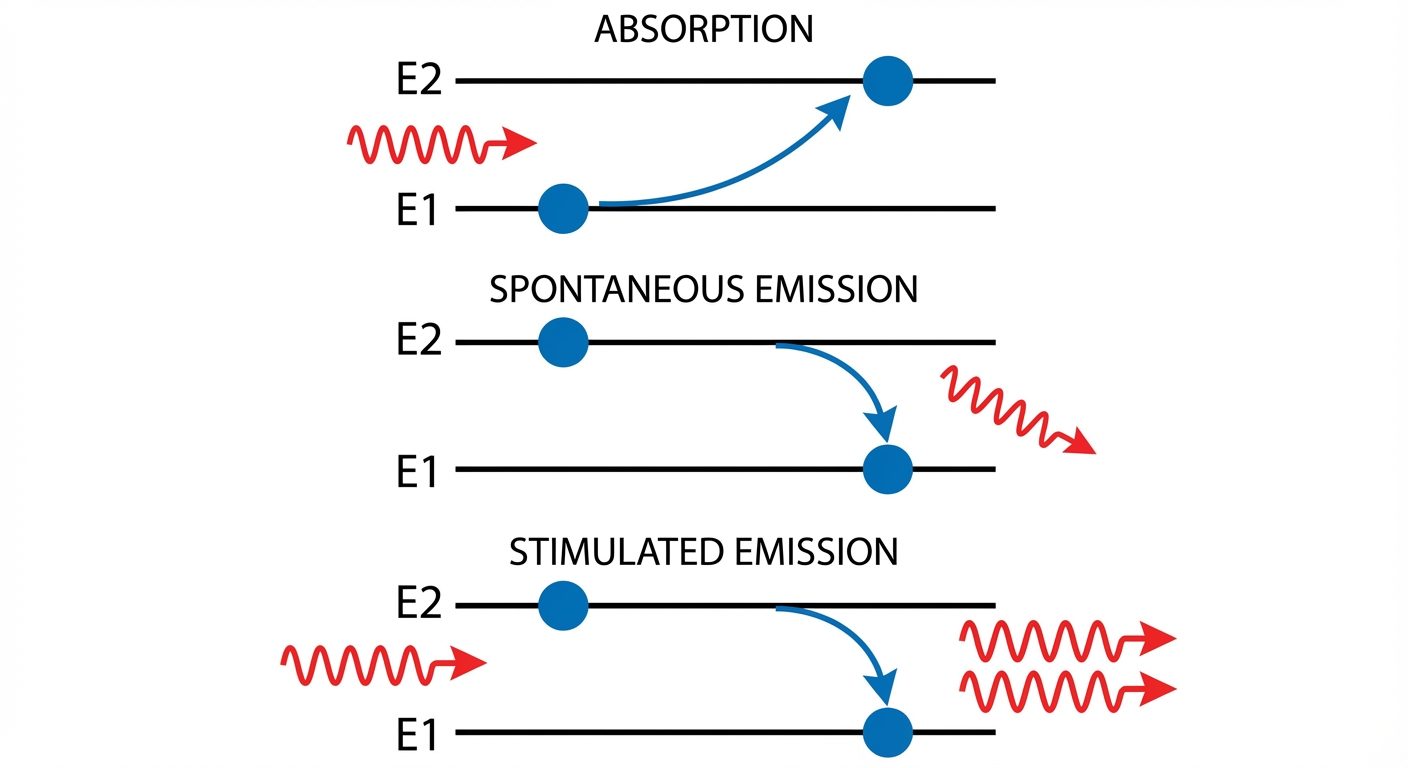
A. Induced Absorption
When an atom in the ground state () absorbs a photon of energy , it moves to the excited state ().
- Equation: (Excited)
B. Spontaneous Emission
An atom in an excited state () is unstable. After a short lifetime (approx ), it naturally decays to the ground state (), releasing the excess energy as a photon.
- Key Characteristic: The emitted photons are random in phase and direction (Incoherent).
- Equation:
C. Stimulated Emission (The principle of Laser)
If an atom is already in an excited state () and is struck by an incident photon of energy , the incident photon triggers the atom to transition down to .
- Result: Two photons are emitted (the incident one and the emitted one).
- Key Characteristic: The two photons are identical in frequency, phase, and direction (Coherent). This leads to light amplification.
2. Einstein Coefficients
Einstein established the mathematical relationship between the probabilities of the three processes described above.
Let be the number of atoms per unit volume in ground state .
Let be the number of atoms per unit volume in excited state .
Let be the energy density of the incident radiation.
- Rate of Absorption:
- Rate of Spontaneous Emission:
- Rate of Stimulated Emission:
Where:
- is the Einstein coefficient for spontaneous emission.
- is the coefficient for absorption.
- is the coefficient for stimulated emission.
At Thermal Equilibrium:
The rate of upward transitions equals the rate of downward transitions.
Using Boltzmann's distribution law () and Planck's radiation law, Einstein derived two major conclusions:
- : The probability of absorption equals the probability of stimulated emission.
- Ratio of Spontaneous to Stimulated:
This implies that spontaneous emission dominates at higher frequencies, making x-ray lasers harder to build than microwave or visible lasers.
3. Essential Conditions for Lasing Action
To achieve laser action (light amplification), three conditions are strictly required:
1. Metastable State
Normally, an excited state lifetime is very short (). A metastable state is a specific excited state where atoms stay for a much longer time (). This "bottleneck" allows atoms to accumulate in the excited state, essential for population inversion.
2. Population Inversion
Under normal thermal equilibrium, the number of atoms in the ground state is higher than in the excited state ().
Population Inversion is the non-equilibrium state where the number of atoms in the excited state exceeds the ground state (). This ensures that stimulated emission (amplification) dominates over absorption.
3. Pumping (Excitation Mechanisms)
The process of supplying energy to the medium to transfer atoms from the lower level to the higher level to achieve population inversion is called pumping.
- Optical Pumping: Using a light source (e.g., Flash lamp in Ruby/Nd:YAG lasers).
- Electrical Discharge: Using high voltage to ionize gas (e.g., He-Ne laser).
- Direct Conversion: Forward bias current (e.g., Semiconductor laser).
4. Resonant Cavity (Optical Resonator)
The active medium is placed between two mirrors:
- Mirror 1: 100% reflecting (High reflector).
- Mirror 2: Partially reflecting (Output coupler, e.g., 90-99% reflective).
Photons bounce back and forth, stimulating more emission on every pass. A small portion escapes through the partial mirror as the laser beam.
4. Types of Lasers
A. Nd-YAG Laser (Solid State Laser)
- Active Medium: Yttrium Aluminum Garnet (YAG - ) crystal doped with Neodymium ions ().
- Type: Four-level laser system.
- Pumping: Optical pumping using a Krypton flash lamp or Diode laser.
- Wavelength: (Infrared).
Working Principle:
- Excitation: Light from the flash lamp excites ions from ground state () to absorption bands ().
- Non-radiative Decay: Ions decay rapidly from to the metastable state (releasing heat, not light).
- Lasing Transition: Population inversion builds at . Stimulated emission occurs from , emitting a photon of .
- Decay: Ions return from to rapidly.
B. Helium-Neon (He-Ne) Laser (Gas Laser)
- Active Medium: Mixture of Helium (10 parts) and Neon (1 part).
- Active Centers: Neon atoms (produce the laser light). Helium acts as an energy transfer agent.
- Type: Four-level laser.
- Pumping: Electrical discharge.
- Wavelength: (Visible Red).
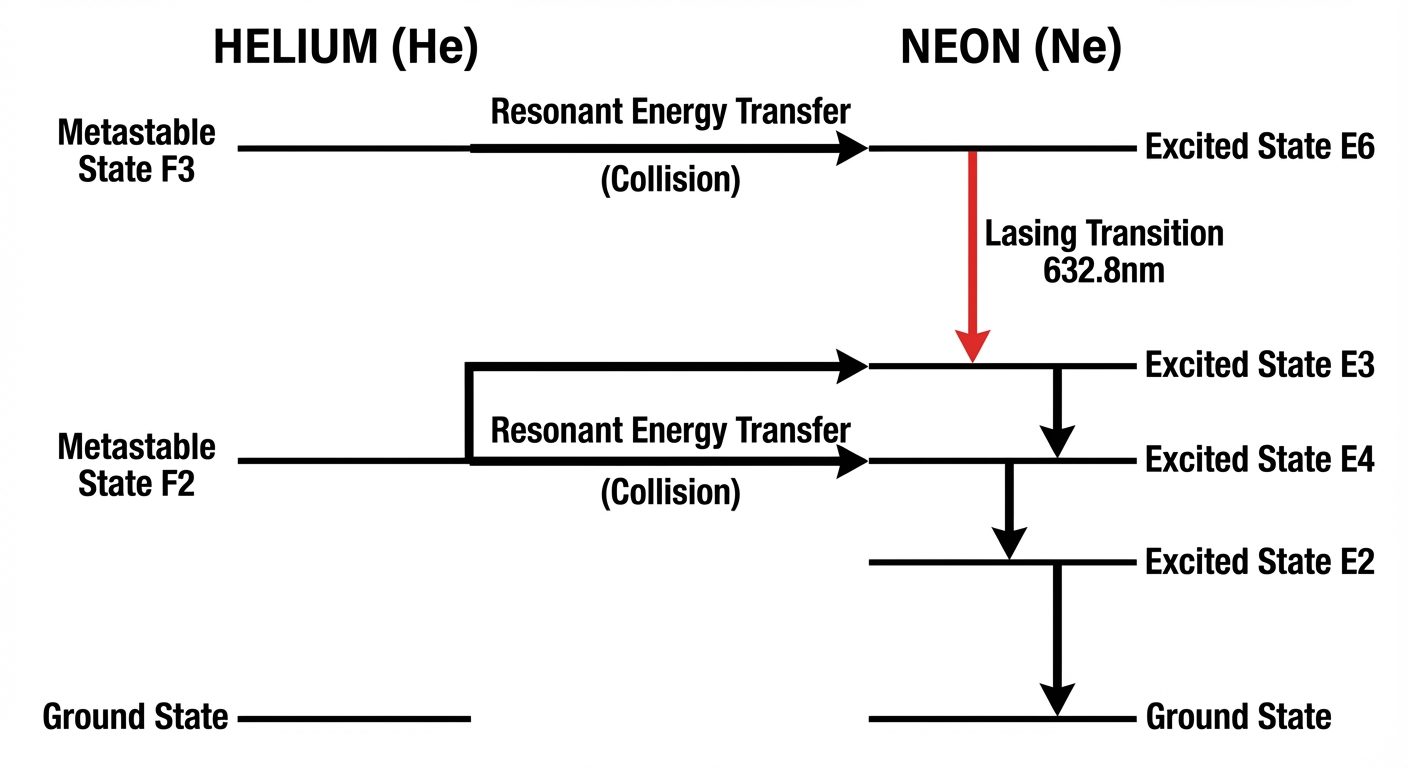
Working Principle:
- Excitation: High voltage accelerates electrons, which collide with He atoms, exciting them to metastable states and .
- Energy Transfer: Excited He atoms collide with Ne atoms. Since He levels match Ne levels ( and ), energy is transferred resonantly to Ne.
- Population Inversion: Ne atoms accumulate in and .
- Lasing: Transition from produces the characteristic red laser light.
- Wall De-excitation: Neon atoms drop to the metastable state and eventually hit the tube walls to return to ground state.
C. Semiconductor (Diode) Laser
- Active Medium: P-N junction diode (usually Gallium Arsenide - GaAs).
- Type: Direct bandgap semiconductor.
- Pumping: Direct conversion (Forward bias voltage).
- Cavity: The polished end faces of the junction act as mirrors (Refractive index difference provides reflection).
Working Principle:
- Heavily doped p-n junction is forward biased.
- Electrons are injected into the Conduction Band (active region) and holes into the Valence Band.
- Recombination: Electrons fall from the conduction band to the valence band, recombining with holes.
- Energy is released as photons ().
- The polished facets reflect photons back into the junction, triggering stimulated emission.
5. Properties of Laser
- Monochromaticity: The laser beam consists of a single wavelength (or a very narrow bandwidth).
- Coherence:
- Temporal Coherence: Phase correlation at a single point over time.
- Spatial Coherence: Phase correlation across different points in the beam cross-section.
- Directionality: Low divergence; the beam travels long distances with minimal spreading.
- High Intensity: Immense energy is concentrated in a very small area.
6. Applications of Laser: Holography
Holography (Greek holos = whole, graphe = writing) is the technique of recording and reconstructing a 3D image of an object. Unlike photography, which records only amplitude (intensity), holography records both amplitude and phase of the light wave.
Basic Principle
Holography is based on the phenomenon of Interference.
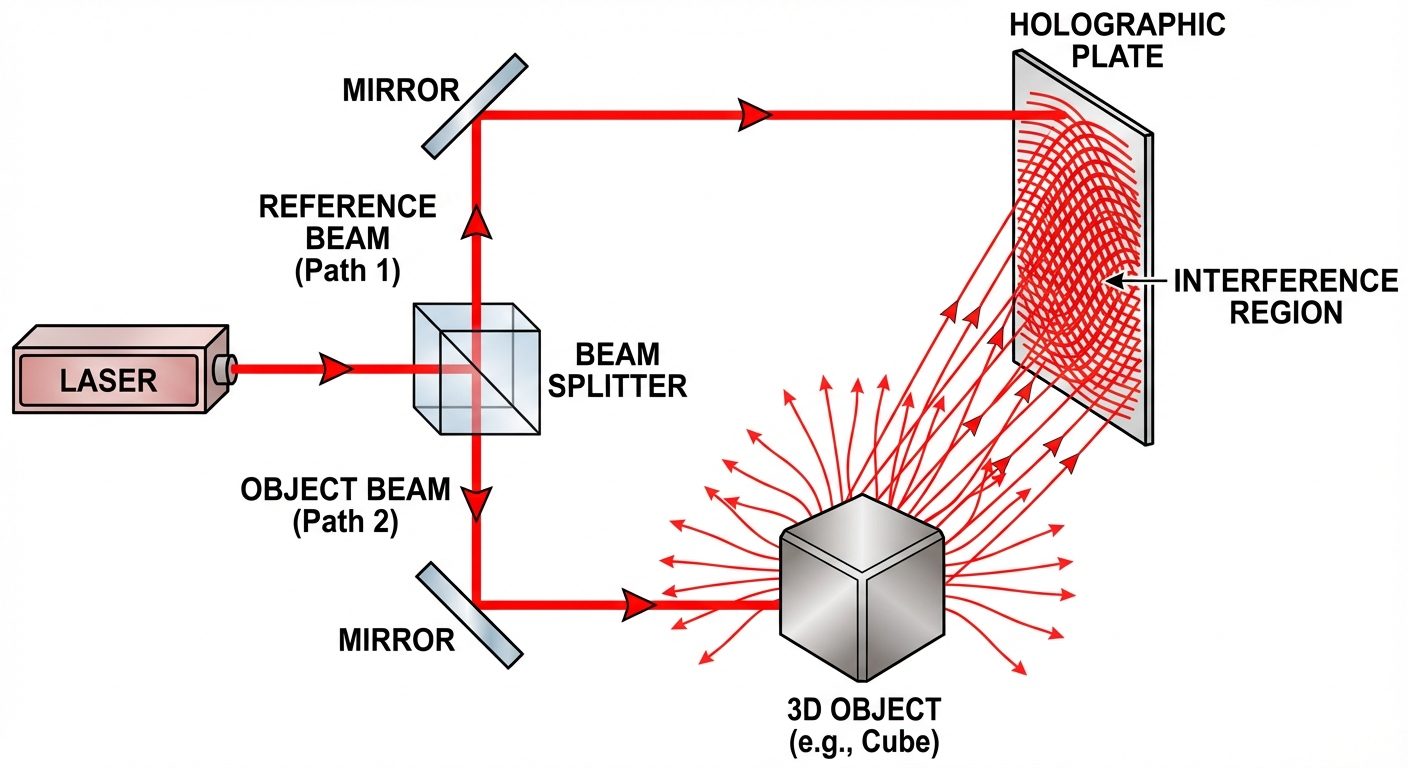
Process 1: Construction (Recording) of the Hologram
- A laser beam is split into two parts by a beam splitter.
- Reference Beam: Goes directly to the photographic plate (holographic plate).
- Object Beam: Illuminates the object, scatters, and then travels to the photographic plate.
- Interference: The two beams (Reference and Object) superimpose on the plate. The phase difference between them creates an interference pattern (fringes) containing the 3D information.
- The developed plate is called a Hologram.
Process 2: Reconstruction of the Image
- The hologram acts as a diffraction grating.
- The developed hologram is illuminated only by the Reference Beam used during recording (at the same angle).
- The light diffracts through the interference pattern.
- Result: Two images are formed:
- Virtual Image: Appears behind the hologram (looks exactly like the original 3D object).
- Real Image: Formed in front of the hologram (can be projected on a screen).
Applications of Holography
- Data Storage: High-capacity holographic memory.
- Security: Holograms on credit cards and currency notes to prevent forgery.
- Interferometry: Detecting microscopic stress and strain in materials.
- Medical: 3D imaging of internal organs.